The Bormed™ Concept
– Because we care.
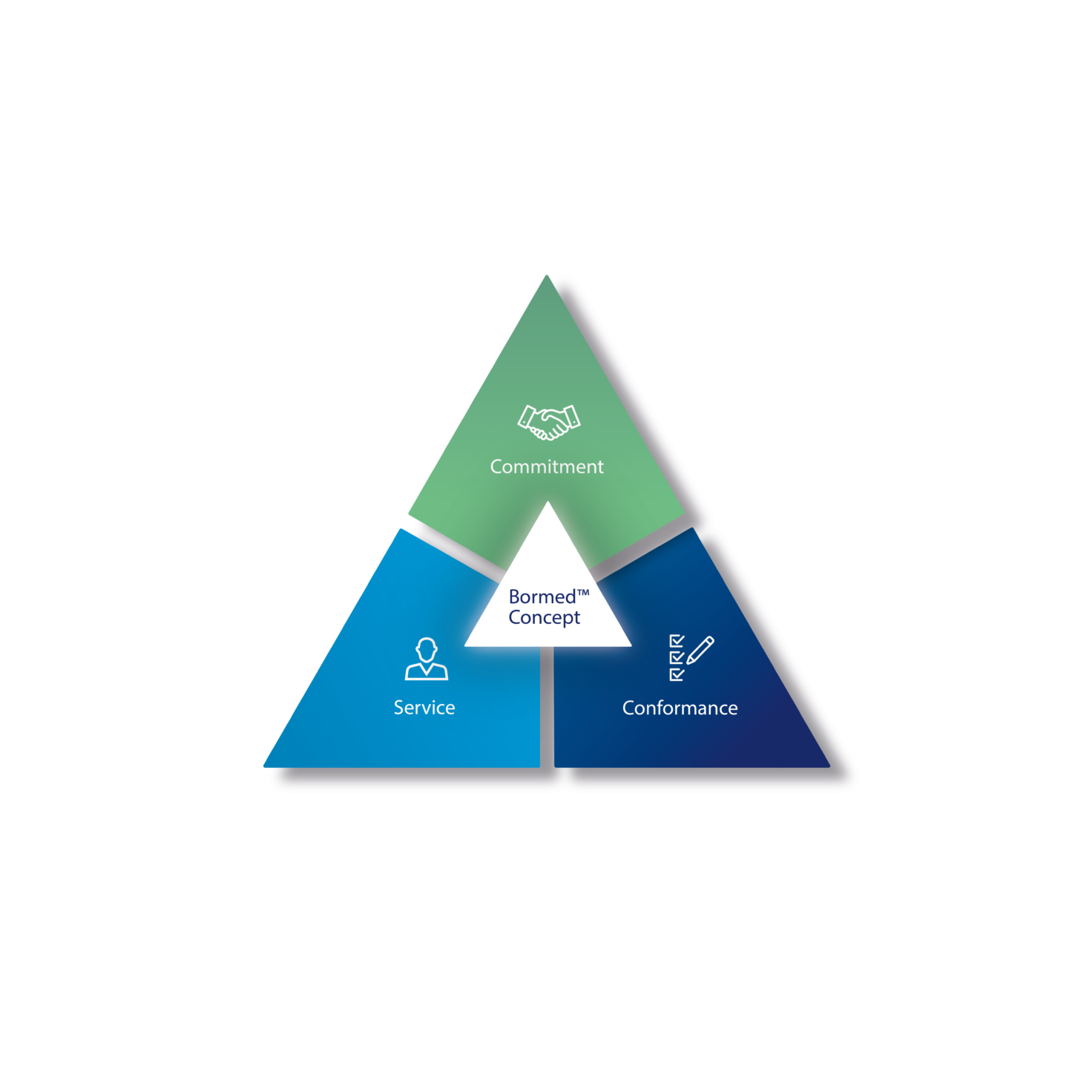
With over 35 years of experience in the healthcare industry, Borealis Bormed™ is founded on the principles of service, commitment and conformance.
-
The Bormed Concept delivers
- Consistency of product recipe via rigorous change control procedure
- Bormed Directive: Operating instructions for the development, production, storage and delivery to the end customer of Bormed
- Continuity of supply to mitigate the risk of a change during your product life cycle: In case of change, product made available up to 5 years (2 years pre-notification and a last call volume combined with 3-year shelf life)
- Pharmacopeia compliance: Regular external testing of Ph. Eur., USP and ISO 10993 – analysis reports can be shared upon request; US DMF listing; following VDI 2017 guideline on “Medical Grade Plastics”
- Externally tested extractable profiles that can be shared upon request (under NDA) and can support your E&L testing programme • Specific technical support given during the project development phase
- Moldflow data and other rheological characteristics
- Globally active dedicated team of experienced technical and regulatory specialists
- Bormed InCompounds: For tailor-made, customised solutions by partnering with trusted and recognised healthcare compounders (at present Avient, MELITEK, MOCOM and Wittenburg Group)

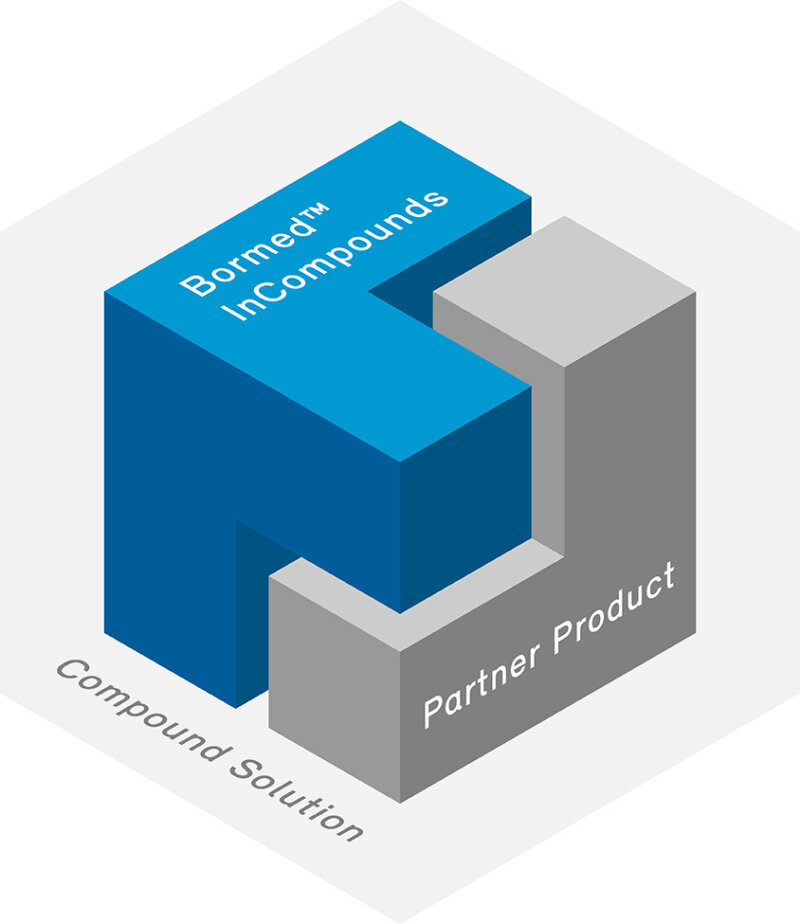
Bormed™ InCompounds
By partnering with trusted and recognized healthcare compounders, we extend the Bormed reach to compound solutions. In doing so we ensure that every end-customer can get the requested tailor-made solution based on Bormed.
Collaborate with us!